Click
on the desired subject.
pH
 ORP
ORP  Conductivity/TDS/Resistivity
Conductivity/TDS/Resistivity  Dissolved Oxygen
Dissolved Oxygen  Turbidimeters
Turbidimeters  Photometers
Photometers

pH
How
often should I calibrate my pH meter ?
This
question brings out another question; how accurate
do you want to be? As no application is the same,
here is a good way to know when calibration is
due:
Procedure
Perform
a calibration on day 1. On the following day,
simply soak your electrode in the original buffers
you performed calibration with and note the readings.
If
the readings are still within your expectations,
keep doing this procedure everyday until you are
not happy with the accuracy. Then proceed to calibrate
and take note of the number of days that went
by since day 1 of calibration. If 5 days went
by, proceed to calibrate every 4-5 days.
As
the pH electrode gets older, proceed to do this
test monthly to confirm number of days required
between calibrations. This is also a good way
to forecast probe
cleaning or replacement.
*All
manufacturers specifications on accuracy are based
on calibration prior to each measurement. Some
specific samples do require calibration prior
to each measurement, such as food products, very
dirty sample or in presence of strong chemical
concentration. Do not hesitate to contact
us for tips about your specific application
or consult this list
of electrode applications.

What
is the expected life of a pH electrode ?
Care
and maintenance is the answer to this question.
Generally speaking, a pH electrode that is well
maintained can last up to 2 years.
High
temperature applications & abrasive chemicals
will greatly shorten the life of a pH electrode.
Pressure is also a factor for in-line process. Make
sure you have the proper
electrode for your application.


What
should I store my pH electrode in and why ?
pH
electrodes need to be kept wet in order to keep
the glass sensitive part in good condition. If the
glass sensitive part dries out for more than 6 months,
the pH electrode will die. If it dries out for a
few months, it can be regenerated by soaking into
storage solution overnight.
The
storage solution brings and keeps a constant ion
activity in the sensing part that will assure fast
response and accurate readings. Always store your
pH electrode after your series of tests are done.
Electrodes that are not stored into storage solution
will show slow response and fluctuations in readings.
NEVER
STORE YOUR pH ELECTRODE INTO DISTILLED WATER.
Many
people think that pure water is good for pH electrodes.
This is wrong. Pure water actually kills pH electrodes
by sucking its electrolyte out of the reference
chamber. Pure water is excellent for rinsing between
samples, but armful for storage purposes. If you
are out of storage solution, a pH 4 buffer can be
used for a few weeks. Long term storage into pH
buffers is not recommended as they contain phosphate.


Why
pH electrodes have different tip shapes ?
pH
is a critical parameter for an incredible number
of applications going from general water to food,
soil, fruits & vegetables, blood, synthetic
products and many others. For that, manufacturers
have developed different pH sensors for all major
applications. This ensures ease of use and longer
life of the electrode in a specific application.
Different types of junctions, electrolytes and materials
used in electrode construction are also part of
the design. Below are typical tips and their intend
:
|
|
Sphere
tip: it is the most common tip found in
the market as it is mainly used in laboratories
on general liquids.
|
 |
Cone
tip: its shape allows easy penetration
into semi solids, emulsion solutions, cheese
and meat. Mainly used in the food industry.
|
 |
Flat
tip: its construction in intended for
surface measurement such as fruits & vegetables
skin, drops of samples, human skin, etc.
|
 |
Knife
tip: the knife probe allows for penetration
into semi-frozen food, meat, hard to penetrate
food products or others.
|
Many
other types of tips are available. The above are the
most common.



 What
is this new ¨Replenishable junction¨ you guys have
invented ?
What
is this new ¨Replenishable junction¨ you guys have
invented ?
Over
time, the junction which is the most sensitive
part of the pH electrode can become clogged. This
results in the electrode response becoming increasingly
sluggish and eventually impossible to calibrate.
With the new Hanna replenishable junctions, by
using an ordinary pair of tweezers, simply pull
out 1-2mm (1/8’’) of the fiber junction
and you will literally have a reconditioned pH
electrode. This procedure can be repeated up to
15 times, before the whole fiber gets out.

What
is the difference between single and double junction
?
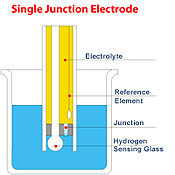 Conventional
electrodes are normally single junction. As
depicted by the figure below, these electrodes
have only a single junction which serves to
put the reference electrode system in contact
with the sample. Under adverse conditions e.g.,
high pressure, high temperature, highly acidic
or alkaline solutions etc., the positive flow
of the electrolyte through the junction is often
reversed resulting in the ingress of sample
solution into the reference compartment. If
this is left unchecked, the reference electrode
ultimately is contaminated, leading to complete
electrode failure. Conventional
electrodes are normally single junction. As
depicted by the figure below, these electrodes
have only a single junction which serves to
put the reference electrode system in contact
with the sample. Under adverse conditions e.g.,
high pressure, high temperature, highly acidic
or alkaline solutions etc., the positive flow
of the electrolyte through the junction is often
reversed resulting in the ingress of sample
solution into the reference compartment. If
this is left unchecked, the reference electrode
ultimately is contaminated, leading to complete
electrode failure. |
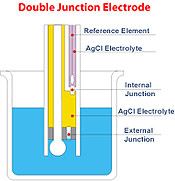
Hanna’s
double junction system, as the name implies,
has two junctions, only one of which is in
contact with the sample. Under adverse conditions,
the same tendency of sample ingress is evident.
However, as the reference electrode system
is separated physically from the intermediate
electrolyte area, the contamination of the
electrode is minimized. This leads to long
electrode life. The chances of recovery are
also higher if proper maintenance procedures
are taken.
|


How
do I measure pH & EC in soil with extraction method?
Extraction
method to measure pH & EC is as follow.
1- Mix 3 oz. of soil with 6 oz. of water
2- Let mixture stand 15-20 minutes
3- Filter liquid into clean cup
4- Measure
Simplify
these pH & EC measurement by measuring directly
into soil with HI 99121 pH meter & HI 993310 EC
meter.


ORP
Do
I need to calibrate an ORP electrode?
No.
ORP electrodes do not need calibration with the
meter such as pH. Still, ORP electrodes need to
be conditioned prior to use.
When
the electrode is new, soak the tip in warm tap water.
This will enhance the flow of the reference junction.
To check function of the electrode, immerse the
tip in ORP solution HI 7021L or HI 7022L. The reading
should be +/- 50mV from the value indicated on the
bottle.
If
the reading is not within the +/-50mV, oxidizing
or reduction treatment with HI 7092 or HI 7091 is
required. It will also prepare the electrode’s
surface and speed initial response time. Since in-line
process electrodes are already in a solution, a
simple test with either HI 7021L or HI 7022L will
show you the electrode’s condition.
Should
your probe not be accurate enough after conditioning
& testing, follow the cleaning
procedure.


ORP
sensor, platinum or gold ?
Platinum
sensor:
use in oxidizing reaction (above 500mV) such as
pools & spas, municipal drinking water.
Gold
sensor:
use in reducing environment (below 500mV) such as
galvanic applications, mining industry (Cyanide).

Conductivity/TDS/Resistivity
What
is the relation between conductivity & dissolved
solids (TDS)?
HOW
By
taking several water samples and making an analysis
of each element in the water and then comparing
the results with the EC measurement, a ratio was
established:
SAMPLE
"X"
| Analysis |
EC
Meter |
Potassium
50 ppm
Magnesium 45 ppm
Calcium 96 ppm
Chloride 80 ppm
Sulphate 99 ppm
Sodium 120 ppm
Aluminum 3 ppm
Zinc 0 ppm
Manganese 1 ppm
Copper 4 ppm
Iron 2 ppm
Boron 0 ppm
Molybdenum 1 ppm
Phosphorus 0 ppm
Nitrate 11 ppm |
|
| TOTAL:
512 ppm |
1000µS
|
|
Most
samples had a ratio of approximately 0.5 for
TDS (ppm) compared to EC (µS/cm). The following
conversion factor was then established:
2
µS/cm = 1 ppm
(2
µS x 0.5 = 1 ppm)
From
there, TDS meters were built to measure conductivity
as usual and then multiply that reading internally
by 0.5 to display a ppm reading.
|
*****
WHY
The
process of making an analysis of each dissolved
element in water is long, expensive and usually
requires experienced people such as chemists. Commercialization
of TDS meters allows any user to have a good approximation
of dissolved solids at an affordable price.
*****
It
is important to know that the conversion factor used
for TDS meters is an approximation of what a complete
analysis would give in TDS.
More
important is the fact that this conversion factor
of 0.5 was set from a general water analysis, containing
normal values of each dissolved element.
For
agricultural application, another type of TDS exist
: TDS442. For detailed explanations of TDS442, click
here.

Dissolved
Oxygen
My
electrode is dry. What should I do?
Remove
the red and black plastic cap or the membrane assembly.
Soak the bottom 1 inch in electrolyte solution for
5 minutes. Rinse the membrane with electrolyte and
refill with clean electrolyte. Gently tap the sides
of the membrane cap to ensure that no air bubbles
remain trapped. Adjust O-Ring inside membrane cap.
With the sensor facing down screw the membrane assembly.
My
readings are not stable. What should I do?
The
probe is under polarization with a fixed voltage of
approximately 800mV. Probe polarization is essential
for stable measurements with the same recurring degree
of accuracy. With the probe properly polarized, oxygen
is continually ''consumed'' by passing through the
sensitive diaphragm and dissolving in the electrolyte
solution contained inside the probe.

Turbidimeters
Why
do I get "Err
1" reading on my turbidity meter?
"Err
1" is an error code that signifies that the light
flow is reduced. The cuvet should be cleaned with
the solution and the tissue designed for this use.
If this procedure has not removed your error code,
the light source will need cleaning. This should be
performed yearly, more frequently if required. The
light source inside the cavity should be cleaned with
the aid of a cotton swab dipped in alcohol.

Photometers
Why
should I do if my readings are unstable?
The
zeroing and the measurements should be done using
the same cuvet. Interferences are possibly due to
condensation or particles on the cuvet wall. Clean
the outside of the cuvet with solution and tissue
designed for this use.